ACF7, as a potential biotherapeutic agent in Malignant Melanoma prestudy in Xenograft Mouse Model.
1. Introduction
Malignant melanoma is one of the most aggressive skin cancers with high metastatic potential and extraordinary resistance to anti-cancer agents [1].
Currently, more than 132,000 melanoma skin cancers occur globally each year [2] without effective chemotherapy against malignant melanoma despite partial success gained by the use of platinum analogues, nitrosoureas, taxanes, Vinca alkaloids and cytokines [3].
Thus, there is a urgent need for developing novel, safe and effective drugs for the treatment of melanoma[4].
Medicinal mushrooms have been used to treat cancer for thousands of years. Unlike Western medicine, which generally uses pure compounds targeting a single molecule or pathway, these mushrooms contain multiple components that interact with multiple molecular targets [5].
There is much anecdotal evidence of the efficacy of mushroom mixtures in cancer patients. However, systematic preclinical evaluation of these mushrooms is necessary. Recently, mushroom mixtures were found to have even stronger activities when using mushroom alone [6].
Phellinus linteus (P. linteus) belongs to the Hymenochaetaceae basidiomycetes that has been used to treat various human cancers in Asia [7].
Inonotus obliquus (IO) is found in the birch forests of Russia, Korea, Eastern and Northern Europe, northern areas and the North Carolina Mountains of the USA and Canada. Numerous studies have revealed its antioxidant, anti-tumor and antimicrobial activities [9].
Antrodia camphorata (AC) is an indigenous and rare mushroom of the Polyporaceae family that only grows on the inner heart rot of the native Taiwanese tree, Cinnamomum kanehirai Hay (Lauraceae). AC is used in traditional Chinese medicine for treating food and drug poisoning, diarrhea, abdominal pain, hypertension, pruritus (skin itch) and liver dysfunction [10].
Ganoderma lucidum ((Leyss. ex Fr.) Karst), a popular medicinal mushroom used for the prevention and treatment of various human diseases, such as hepatitis, hypertension, chronic bronchitis, bronchial asthma, cancer and others in China [13].
Numerous studies have described that the major bioactive components in G. lucidum are polysaccharides, ganoderic acid (triterpene) and adenosine [14].
We have evaluated various formulations of mushroom mixtures to choose the most effective one against B16F10 mouse melanoma cells in vitro. Based on preliminary results, we decided to further evaluate the effects of ACF7 (Antitumor Compounds of Fraction 7), the extraction composed of Phellinus linteus, Inonotus obliquus, Antrodia camphorata and Ganoderma lucidum.
In previous study, we have evaluated the effects of mushroom mixture ACF7 on the growth and invasive behavior of B16F10 melanoma cells in xenograft-mouse models.
The body weight and general appearance of each mouse were measured and observed every day as evidence of systemic toxicity. In the acute toxicity study, doses up to 1,000 mg/kg did not exhibit any mortality or any signs of toxicity (e.g., changes in body weight and the amount of food consumption) after oral administration of a single dose (Figure 1). This dose may be considered as the no observed adverse effect level (NOAEL) [15] for ACF7.
Furthermore, the toxicity guideline [16] discourages studies beyond 1 g/kg if no abnormality is observed.
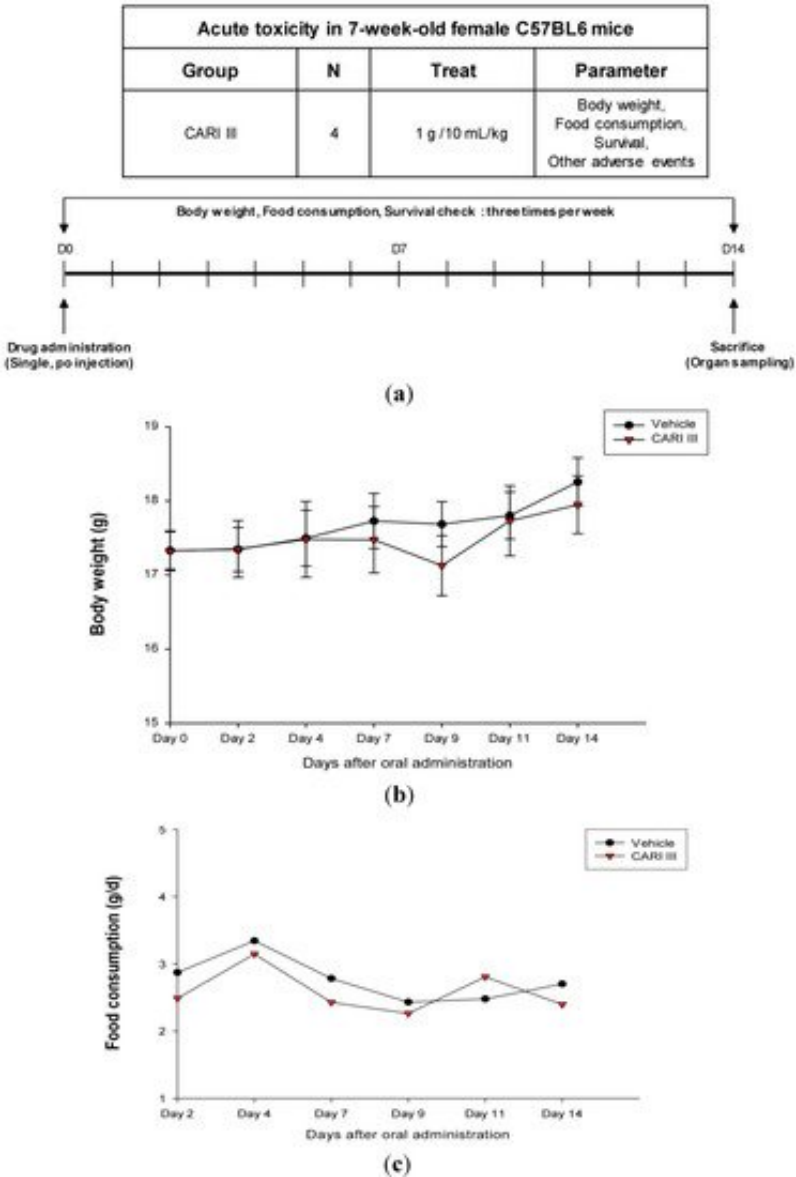
Figure 1. The effect of ACF7 on body weight and food consumption in the acute toxicity study. (a) Scheme of the acute toxicity study. (b) Body weight is presented as the mean ± SE (bars). Average diet consumption per mouse per day is presented once a day. The two groups represented below are the control group and the ACF7 group (1000 mg/kg). (c) Daily food intake consumption.
We examined whether ACF7 could inhibit tumor growth in vivo. Intraperitoneal solid tumors developed in C57BL6 mice by intraperitoneal injection of B16F10. ACF7 or saline was administered orally three days before tumor cell implantation (Figure 2). Mean tumor weights were significantly suppressed in ACF7-treated mice (300 mg/kg/day) compared to tumor controls. Doxorubicin (Dox) used as a positive control (1 mg/kg i.p. once daily for 17 days) also significantly inhibited tumor growth and tumor weight (p < 0.001 and p < 0.001, Figure 3). Doxorubicin (Dox) is one of the most widely used antineoplastic drugs against many solid tumors, including melanoma. Although Dox has been shown to have potent antitumor activity, its effectiveness is often limited by drug resistance and dose-dependent side effects, especially Dox-induced cardiomyopathy [17]. The study showed that the 300 mg ACF7 III/kg/day regimen reduced tumor weight compared to the doxorubicin (Dox) treatment group.
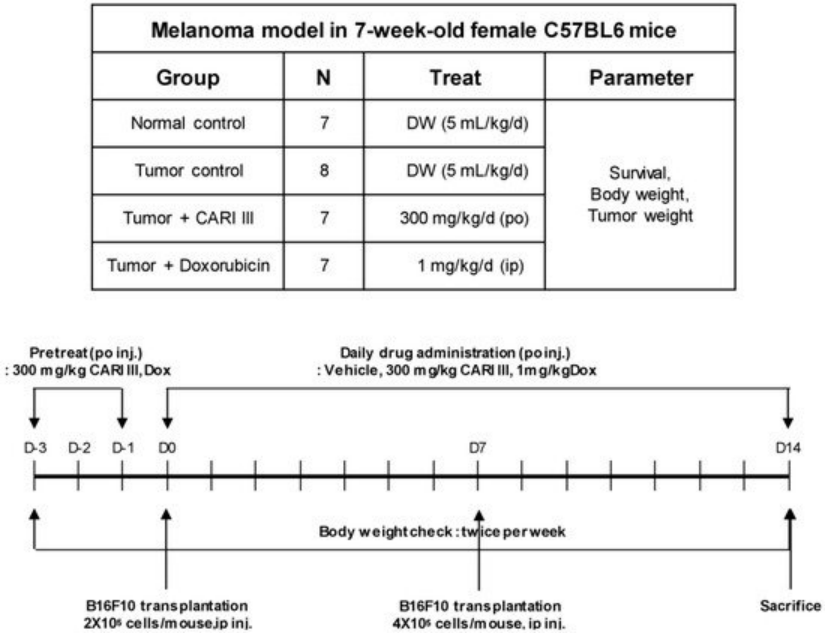
Figure 2. Experimental design of the effect of ACF7 or doxorubicin (Dox) on B16F10 melanoma xenograft in an animal model.
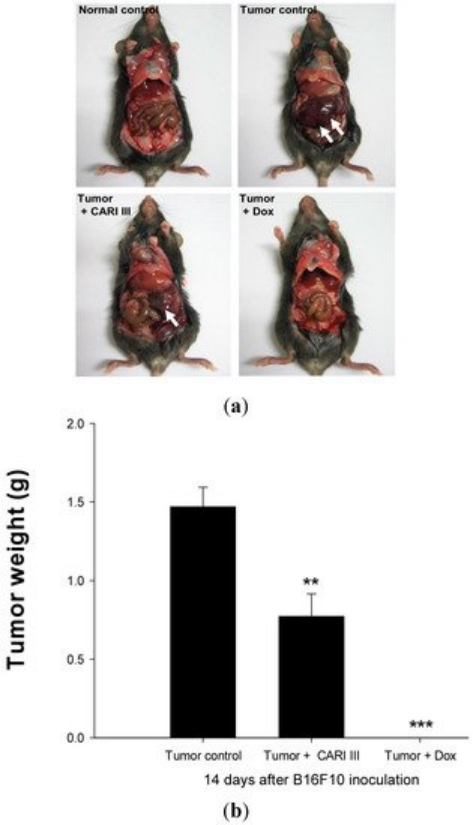
Figure 3. The effect of ACF7 on tumor growth in B16F10 melanoma-bearing mice. (a) Representative mice were administered with ACF7, doxorubicin (1 mg/kg/day) or DW (distilled water). Mice were sacrificed on Day 14 after B16F10 inoculation. (b) Tumor weight changes on B16F10-inoculated mice treated with DW, ACF7 (300 mg/kg/day) and Dox (1 mg/kg/day). Data were obtained from the three independent experiments and were expressed as the means ± SE. One-way ANOVA was used for the comparison of multiple group means, followed by Dunnett’s t-test (** p < 0.01, *** p < 0.001 vs. Tumor control).
The effects of ACF7 on the survival rate of mice are shown in Table 1. The survival rate of mice treated with ACF7 was prolonged compared to that of the tumor control group. Survival rates were 57% and 86%, for the DW (distilled water)- and ACF7 -treated group, respectively.
A Kaplan–Meier plot illustrates that the survival rate of ACF7 extract-treated mice was increased when compared to tumor control animals. The maximum survival rate was observed in the ACF7-administered group, as shown in Figure 4.
The percentage increase in life span = T − C / C × 100, where T and C are the percent of surviving mice in the treated and tumor control groups. An increase in life span (ILS% = 50.88%) was obtained in the ACF7 -administered group, compared to the tumor control group.
Table 1. ACF7 increases the survival rate of B16F10 melanoma-bearing mice.
Table 1. ACF7 III increases the survival rate of B16F10 melanoma-bearing mice. |
Group | Initial | Final | Death | Survival Rate (%) at Day 14 |
Normal control | 6 | 6 | 0 | 100 |
Tumor control | 6 | 4 | 3 | 57 |
Tumor + ACF7 | 7 | 6 | 1 | 86 |
Tumor + Dox | 7 | 7 | 0 | 100 |
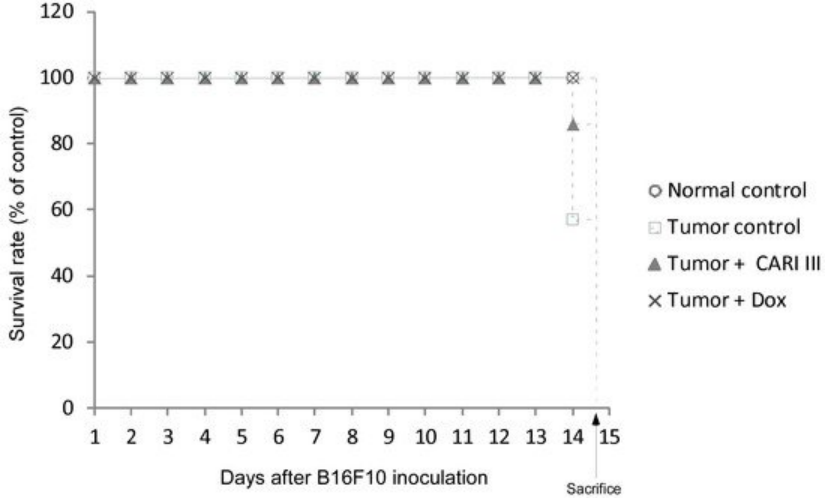
Figure 4. ACF7 increases the survival rate of B16F10 melanoma-bearing mice. Kaplan–Meier curves for B16F10 murine neoplasms in C57BL/6 mice. The number of animals per group was seven (n = 7).
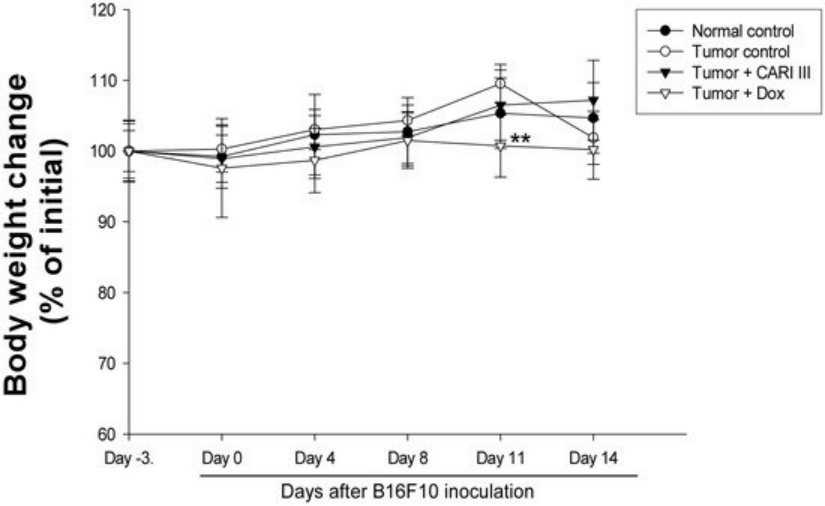
Figure 5. Effect of ACF7 on body weight. The body weight of the mice was measured during treatment. The mean body weights before ACF7 treatment in each group are expressed as 100%. The body weight of the following days is expressed as the ratio of the body weight to the initial body weight. Data were obtained from the three independent experiments and were expressed as the means ± SE. One-way ANOVA was used for the comparison of multiple group means, followed by Dunnett’s t-test (** p < 0.01 vs. Tumor control).
The body weights of the ACF7-treated mice were similar to those of the normal control group (Figure 5), indicating that there were no significant side effects during treatments. Data indicate that melanoma growth was significantly suppressed in mice treated with ACF7 without loss of body weight, while the Dox-treated mice showed significant body weight loss. Based on the observation of body weight (Figure 5), a small amount of host toxicities were observed after Dox treatment.
We also evaluated if the mixture of mushrooms also could inhibit the growth of highly invasive B16F10 cancer cells in vitro. As seen in Figure 6, ACF7 markedly suppressed proliferation of B16F10 cells at 250 and 500 μg/mL (Figure 6) (p < 0.05 vs. control).
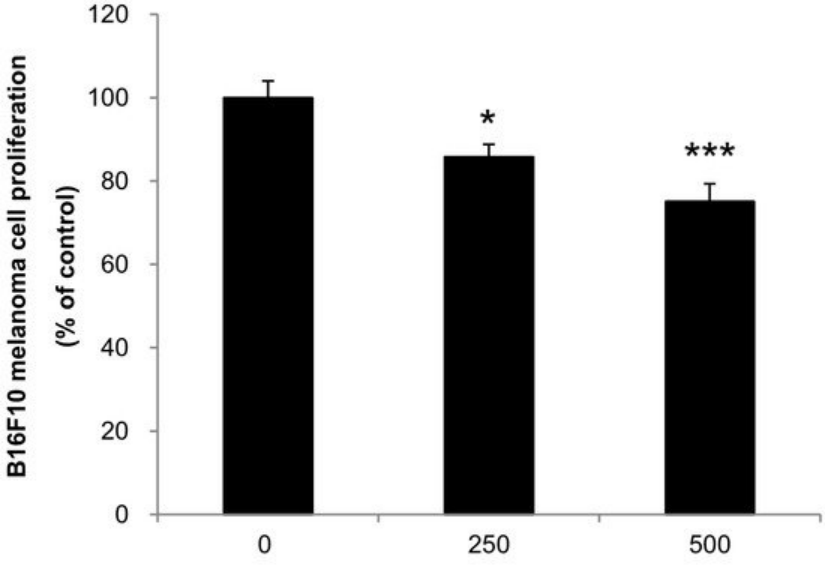
Figure 6. Anti-proliferative effects of ACF7 extracts against B16F10 melanoma. Cells were treated with the indicated concentrations of ACF7 III extracts for 72 h. Cell viability was compared to the control, and the statistically different levels are denoted by: * p < 0.05, *** p < 0.001. Data were obtained from the three independent experiments and are expressed as the means ± SE.
3. Experimental
3.1. Preparation of ACF7
ACF7, composed of Phellinus linteus (PL), Inonotus obliquus (IO), Antrodia camphorata (AC) and Ganoderma lucidum (GL), was supplied by 金源佳和. were mixed as appropriate weight ratio and broken into powder. The powder was extracted with 80% ethanol (EtOH) for 48 h. The residue was extracted at room temperature and filtered again. The extract was dried by a rotary evaporator under vacuum at 40 °C and stored at −20 °C until use. ACF7, the mixture of PL, IO, ACand GL, was dissolved in water and used for the animal experiments.
3.2. Experimental Animals
Female C57BL/6 mice at 6 weeks of age and acclimatized under the controlled conditions for 1 week before the experiment.
3.3. Acute Toxicity Test
ACF7 was given orally to mice (12 mice/group) at single doses of 1000 mg/kg/day along with the vehicle control. Animals were observed during the first 12 h for any alteration in the symptoms of mobility, posture, the amount of food consumption and for mortality. Mice were weighed daily and observed for fourteen days following treatment.
3.4. Tumor Growth Analysis in Vivo
The inhibitory effect of ACF7 on melanoma tumor growth was investigated in an animal model. Tumors were induced as previously described [12]. Experiments were performed in six groups: normal control group, saline injection; tumor control group, B16F10 (2 × 105 cells/mouse) injected intraperitoneally (i.p.); ACF7 treated group, B16F10 (2 × 105 cells/mouse) injected intraperitoneally; ACF7 III oral administered group, B16F10 (2 × 105 cells/mouse) injected intraperitoneally; and doxorubicin (Dox) (Sigma, St. Louis, MO, USA) intraperitoneally injected group, B16F10 (2 × 105 cells/mouse) (n = 7 per group). ACF7 (300 mg/kg/day) and doxorubicin (1 mg/kg/day) were administered three days before B16F10 melanoma cell transplantation until sacrifice, respectively.
Body weight was measured once every three days. The number of surviving animals was examined every day. Tumor was analyzed on Day 14 following B16F10 melanoma cell transplantation. Mice were sacrificed 14 days following cell inoculation. Tumor tissues were collected for further analysis. Tumor was imaged with a digital camera.
3.5. Cell Culture
The B16F10 melanoma cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in MEM (Invitrogen Co., Carlsbad, CA, USA) containing 10% (v/v) fetal bovine serum, 100 unit/mL penicillin and 100 µg/mL streptomycin in an atmosphere of 95% air and 5% CO2 at 37 °C.
3.6. Cell Proliferation Assay
Cell proliferation was measured using the Cell Counting Kit-8 (CCK-8) assay (Dojindo Laboratories, Kumamoto, Japan), as described previously [18]. Cells (1 × 104 cells/well) were incubated with the indicated concentrations in the absence or presence of samples for the indicated times. The cell proliferation rate (%) was calculated as the absorbance of sample-treated cells divided by the absorbance of control cells (n = 3). The cell viability of the control group was calculated as 100%.
3.9. Statistical Analysis
Data are expressed as means ± standard error of the mean (SEM). Differences between groups were determined by one-way ANOVA followed by Dunnett’s t-test. Significant differences were considered from * p < 0.05, ** p < 0.01,*** p < 0.001.
4. Conclusions
The results has demonstrated that ACF7 exerted anti-cancer activity that is comparable to Dox against melanoma in vitro and in vivo. ACF7 exerts inhibitory effect on the proliferation of B16F10 melanoma cells. The 300 mg ACF7/kg/day regimen reduced tumor weight, comparable to the doxorubicin (Dox)-treated group, along with increased life span.
These data suggest that ACF7 might be a promising anti-cancer agent against melanoma. Further study will focus on the molecular mechanism of ACF7 in vivo comprehensively.
References
00001. Hoang, M.T.; Eichenfield, L.F. The rising incidence of melanoma in children and adolescents. Dermatol. Nurs. 2000, 12, 188–189, 192–193. [Google Scholar]
00002. Navarini, A.L.; Chiaradia, L.D.; Mascarello, A.; Fritzen, M.; Nunes, R.J.; Yunes, R.A.; Creczynski-Pasa, T.B. Hydroxychalcones induce apoptosis in B16-F10 melanoma cells via GSH and ATP depletion. Eur. J. Med. Chem. 2009, 44, 1630–1637. [Google Scholar]
00003. Sun, W.; Schuchter, L.M. Metastatic melanoma. Curr. Treat. Options Oncol. 2001, 2, 193–202. [Google Scholar]
00004. Harhaji, L.; Mijatovic, S.; Maksimovic-Ivanic, D.; Stojanovic, I.; Momcilovic, M.; Maksimovic, V.; Tufegdzic, S.; Marjanovic, Z.; Mostarica-Stojkovic, M.; Vucinic, Z.; et al. Anti-tumor effect of Coriolus versicolor methanol extract against mouse B16 melanoma cells: In vitro and in vivo study. Food Chem. Toxicol. 2008, 46, 1825–1833. [Google Scholar] [CrossRef]
00005. Patel, S.; Goyal, A. Recent developments in mushrooms as anti-cancer therapeutics: A review. 3 Biotech 2012, 2, 1–15. [Google Scholar]
00006. Vetvicka, V.; Vetvickova, J. Immune enhancing effects of WB365, a novel combination of Ashwagandha (Withania somnifera) and Maitake (Grifola frondosa) extracts. N. Am. J. Med. Sci. 2011, 3, 320–324. [Google Scholar]
00007. Lee, I.K.; Yun, B.S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011, 64, 349–359. [Google Scholar] [CrossRef]
00008. Park, H.J.; Choi, S.Y.; Hong, S.M.; Hwang, S.G.; Park, D.K. The ethyl acetate extract of Phellinus linteus grown on germinated brown rice induces G0/G1 cell cycle arrest and apoptosis in human colon carcinoma HT29 cells. Phytother. Res. 2010, 24, 1019–1026. [Google Scholar]
00009. Kim, Y.O.; Han, S.B.; Lee, H.W.; Ahn, H.J.; Yoon, Y.D.; Jung, J.K.; Kim, H.M.; Shin, C.S. Immuno-stimulating effect of the endo-polysaccharide produced by submerged culture of Inonotus obliquus. Life Sci. 2005, 77, 2438–2456. [Google Scholar]
00010. Geethangili, M.; Tzeng, Y.M. Review of Pharmacological Effects of Antrodia camphorata and Its Bioactive Compounds. Evid. Based Complement. Altern. Med. 2011, 2011. Article ID 212641. [Google Scholar] [CrossRef]
00011. Park, D.K.; Park, H.J. Ethanol extract of Antrodia camphorata grown on germinated brown rice suppresses inflammatory responses in mice with acute DSS-induced colitis. Evid. Based Complement. Altern. Med. 2013, 2013. Article ID 914524. [Google Scholar] [CrossRef]
00012. Song, M.; Park, D.K.; Park, H.J. Antrodia camphorata grown on germinated brown rice suppresses melanoma cell proliferation by inducing apoptosis and cell differentiation and tumor growth. Evid. Based Complement. Altern. Med. 2013, 2013. Article ID 321096. [Google Scholar] [CrossRef]
00013. Boh, B.; Berovic, M.; Zhang, J.; Zhi-Bin, L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007, 13, 265–301. [Google Scholar]
00014. Sanodiya, B.S.; Thakur, G.S.; Baghel, R.K.; Prasad, G.B.; Bisen, P.S. Ganoderma lucidum: A potent pharmacological macrofungus. Curr. Pharm. Biotechnol. 2009, 10, 717–742. [Google Scholar]
00015. Alexeeff, G.V.; Broadwin, R.; Liaw, J.; Dawson, S.V. Characterization of the LOAEL-to-NOAEL uncertainty factor for mild adverse effects from acute inhalation exposures. Regul. Toxicol. Pharmacol. 2002, 36, 96–105. [Google Scholar]